Ready to Upgrade Your Process Operations?
Create a compliant, scalable cosmetics business plan with MoCRA guidance, manufacturing strategy, QA controls, and financial planning for predictable production.
.jpg)
The U.S. beauty and personal care market is set to exceed $130 billion by 2025 and reach over $140 to $150 billion by 2030, according to a Mordor Intelligence study. The opportunity is real, but so is the pressure.
New brands often face the same challenge you may be dealing with now: maintaining compliance, choosing the right manufacturer, controlling costs, and keeping timelines steady while using MoCRA. It is easy for teams to feel stretched, especially when supplier delays or documentation issues threaten launch dates.
A clear cosmetics business plan brings order to this complexity. It protects your capital, strengthens decision-making, and helps you avoid the operational risks that slow most new brands.
This guide focuses on the operational sections that matter most for U.S.-based brands. It explains how to build a plan that aligns with MoCRA rules, cGMP expectations, quality requirements, and cost management, so your idea can grow into a predictable production schedule in 2026.
The Executive Summary introduces the brand and explains how you plan to maintain compliance and operational stability throughout production.
Include the following:
Securing a U.S.-based, cGMP-aligned manufacturer can be positioned as a key achievement because it stabilizes early production planning.
Note: For brands prioritizing low supplier risk, securing an FDA-registered manufacturer like Respect Manufacturing should be listed as a key operational milestone in the summary.
Once the foundation is clear, the next step is to show that the product has market demand and can scale without stressing procurement and production systems.
This section confirms that your product concept matches real market needs and aligns with realistic throughput expectations.
Describe product characteristics in a way that helps operations and quality teams understand manufacturing needs:
The US cosmetics market is a multi-billion-dollar field. Your plan must identify and estimate your SOM and one-year production volumes.
Market Sizing and Operational Relevance
Also Read: How to Start a Makeup Line: A Step-by-Step Guide
Outline planned SKUs, packaging formats (bottles, jars, tubes, or sachets), and any services (e.g., refills or subscription bundles). Focus on manufacturability, testing requirements, and component lead times.
Identify your priority customer groups and link each to expected buying behavior, typical reorder cycles, and preferred price ranges. This strengthens forecasting for SOM and throughput.
Once market potential and customer alignment are established, the plan must demonstrate compliance with the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) to make sure that products can be legally sold.
MoCRA introduced new expectations for manufacturers and Responsible Persons. Your business plan must show readiness in each area to stand out in 2026.
Brands must rely on manufacturers that maintain:
Brands must file:
Failure in any of these items can pause legal shipments and stop throughput instantly.
Also Read: The Ultimate MOCRA Guide 2025: FDA Requirements & Timelines
Manufacturers should operate with cGMP-aligned procedures and documented testing, including:
Brands must report serious adverse events within 15 business days. Records must be kept for up to six years or three years for qualifying small businesses. Your plan should describe how you and the manufacturer will track documentation for investigations and reporting.
Is your potential manufacturer MoCRA-ready? Review our checklist for mandatory facility registration and cGMP compliance before signing a contract. Contact Respect Manufacturing to review your compliance credentials.
Once regulatory compliance is mapped, the next stage is to explain how production and supply chain activities will operate.
This section outlines how products will be produced, inspected, packaged, and shipped. It strengthens credibility for both investors and manufacturing partners.
Use this table to understand how each model affects operations.
Turnkey vs Toll Manufacturing and KPI Impact
A business plan must detail the QA steps taken before the product leaves the facility. These must include the following:
These controls reduce scrap rate and support MoCRA safety substantiation.
Strong logistics and inventory planning support lead time stability, protect throughput, and make sure that products move smoothly from production to distribution. While creating the business plan, you need to discuss the following:
With operations defined, the next priority is aligning financial projections with realistic manufacturing and distribution data.
Investors scrutinize the financial section to assess the scalability and profitability of the cosmetic business plan. Avoid vanity metrics and focus on operational finance. This section demonstrates the economic stability of your plan.
This must clearly itemize the initial investment required to go from concept to first shipment. The following should be dealt with in your plan:
The projections must be realistic, based on your SOM analysis and verifiable manufacturing costs. Focus on realistic metrics:
Financial goals must be paired with a sales plan that supports consistent throughput.
Are your current COGS projections inflating your CPU? Utilize a turnkey model to consolidate procurement and manufacturing overhead, maximizing profitability. Request a Transparent COGS Breakdown from Respect Manufacturing.
This section explains market entry and growth plans while maintaining compliance and operational feasibility.
Define the go-to-market path and associated logistical requirements:
Each channel affects manufacturer uptime and inventory planning.
Marketing must comply with the FDA's definition of a cosmetic vs. a drug.
The strategy now needs a team structure that can manage compliance, vendor communication, and production monitoring.
Your business plan should also introduce the people behind the work. Share a quick overview of who handles R&D, quality, regulatory tasks, procurement, and day-to-day operations.
This usually includes the Founder or CEO, a Product Development Lead, an Operations Manager, and someone responsible for quality or regulatory checks. Keep it simple and explain how this team will communicate with the manufacturer and review production timelines.
Also Read: How to Start a Skincare Line in 2025
Some brands use the following model to organize production, compliance documentation, and quality planning. Respect Manufacturing operates as a U.S.-based, FDA-registered, cGMP-aligned turnkey partner that manages formulation, packaging, testing, and production under one workflow. This structure helps brands maintain consistent throughput, reduce supplier risk, and improve overall Lead Time Stability.
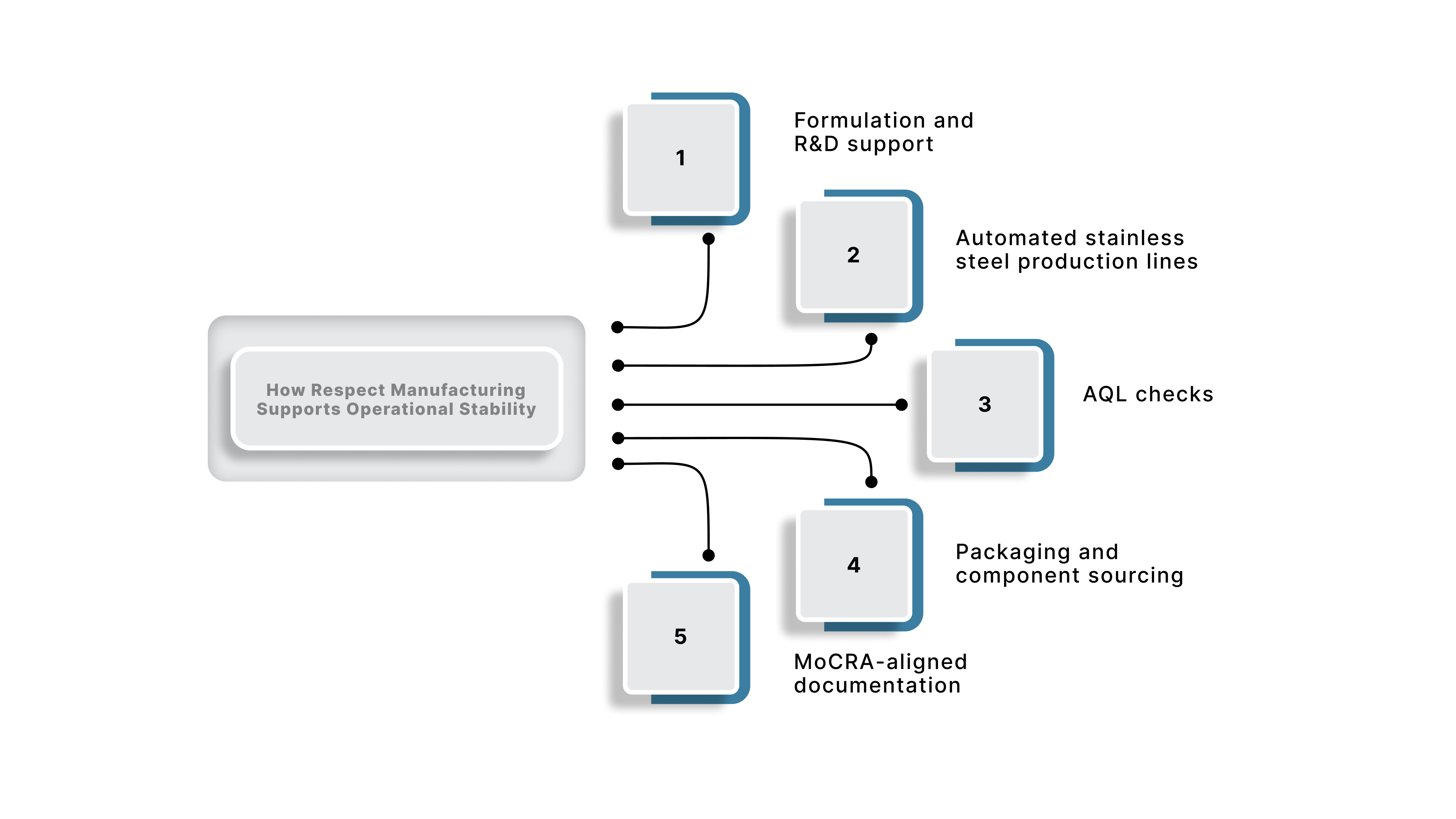
Key strengths include:
This type of partner model gives operations and procurement teams a clear benchmark for what reliable turnkey manufacturing can look like in practice.
A complete cosmetics business plan connects concept, compliance, and scalable production. Working with a U.S.-based, cGMP-aligned turnkey manufacturer supports traceability, stability testing, packaging control, inspection standards, and predictable throughput. This gives brands a stronger foundation to grow and maintain compliance under MoCRA.
If you are preparing to scale your product line in the U.S. market, contact Respect Manufacturing, which supports formulation, packaging, testing, and production under a turnkey model aligned with MoCRA and cGMP expectations.
A cosmetics business plan should confirm your manufacturer's FEI status, facility registration, product listings, cGMP-aligned workflows, safety substantiation, and adverse event documentation. These items make sure that your products can be legally sold in the United States.
Select a manufacturer with FDA registration, cGMP-aligned processes, AQL inspection systems, full traceability, and reliable Lead Time Stability. Your cosmetics business plan should also compare turnkey and toll models based on CPU, supplier risk, and throughput needs.
SOM, or Serviceable Obtainable Market, helps you estimate the sales volume your team can realistically achieve. This metric informs throughput planning, packaging forecasts, and CPU calculations, which are key for operational forecasting in a cosmetics business plan.
A strong cosmetics business plan includes CPU and COGS calculations, break-even volumes, working capital requirements, MOQs, freight costs, and 3- to 5-year forecasts based on verified production data and realistic marketing budgets.
A supports consistent product performance and compliance. Your cosmetics business plan should outline batch record reviews, AQL checks, stability testing, COA verification, and traceability systems that help reduce scrap rate and support MoCRA safety substantiation.



