Ready to Upgrade Your Process Operations?
Master FDA cosmetic labeling with our guide. Avoid misbranding, meet principal display panel requirements, and ensure ingredient compliance. Shop now!

Ever picked up a skincare bottle and wondered what all that fine print really means? From “active ingredients” to “net contents” and those mysterious batch numbers, every word on that label tells a story.
However, for a founder bringing their first cosmetic line to life, that story isn’t just creative; it’s regulatory. One misplaced phrase, one missing disclaimer, and your dream product could hit a legal roadblock before it ever reaches a shelf.
With the global beauty market projected to grow nearly 5% annually through 2030, experts agree that compliance and precision will set “me-too” launches apart from breakout brands. That’s why understanding FDA cosmetic labeling isn’t just a box to tick; it’s the foundation of your brand’s credibility.
In this guide, you'll learn what the FDA expects, common mistakes new beauty founders make, and how the right manufacturing partner keeps you compliant without losing your brand’s voice.
Key Takeaways:
Before your product can make it to the shelf, it needs to meet how the U.S. Food and Drug Administration (FDA) defines a cosmetic, and that starts with intended use.
Here’s what you need to know:
Here’s a quick look at what this means for your brand:
Once you’ve grasped the FDA’s labeling framework, the next step is knowing where each element belongs.
A cosmetic label is more than just artwork; it’s a silent conversation with both consumers and regulators.
The front, known as the Principal Display Panel (PDP), introduces the brand, the product’s purpose, and its visual appeal. The back or side, called the Information Panel (IP), holds the facts: ingredients, directions, warnings, and who stands behind the product.
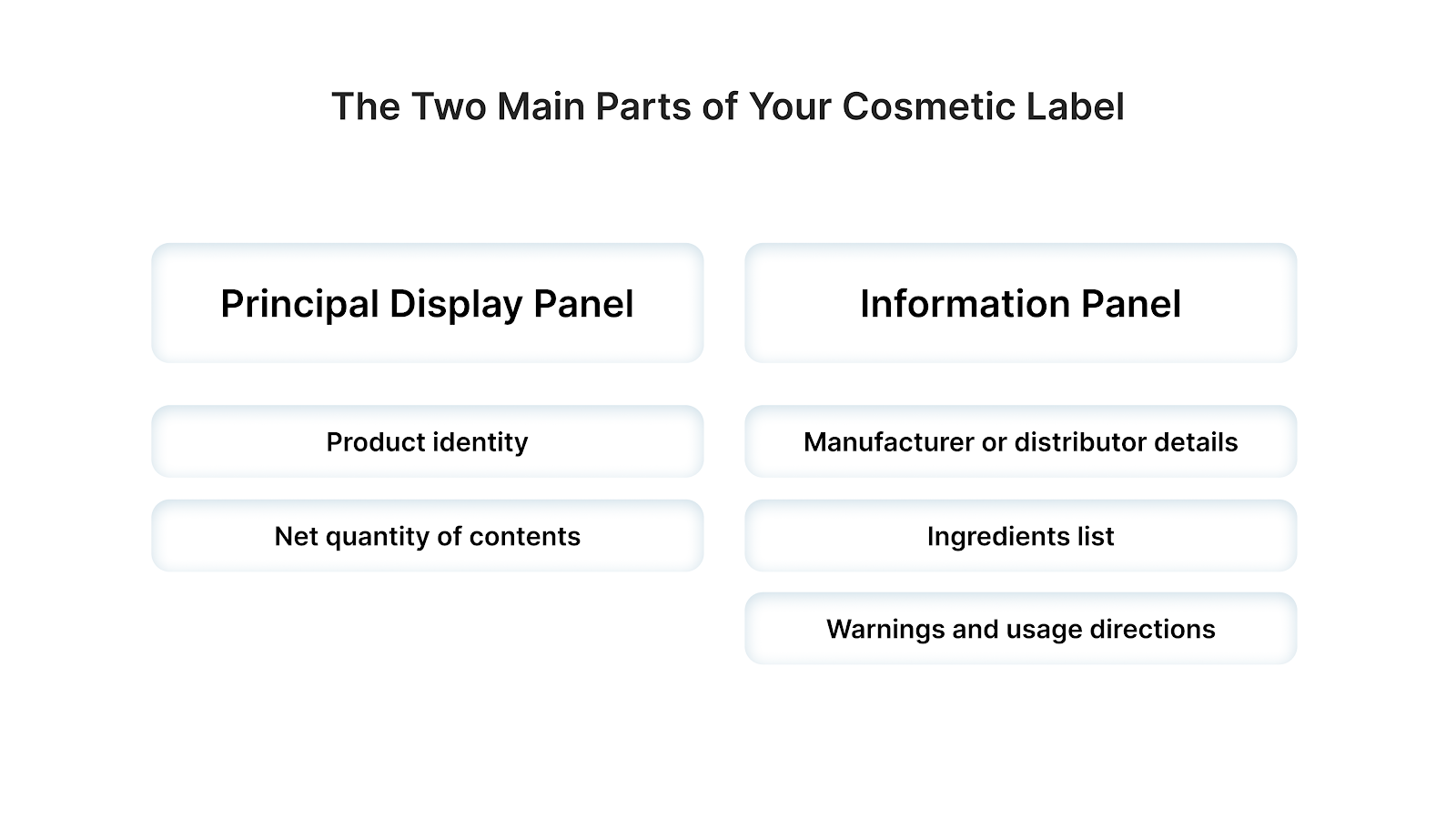
The PDP draws attention; the IP earns trust. Together, they create a label that is both compliant and credible. To understand how each part works and what it must include, let’s take a closer look at both.
This is the front face of your product; the part customers actually see first on a store shelf or in an online thumbnail. Think of it as your brand’s handshake.
Here’s what must go on the PDP:
A few things people often miss:
In short: Your PDP isn’t just about aesthetics, it’s a legal requirement and your first marketing impression. A clean, compliant front panel builds instant credibility.
This one usually sits on the back or side of your product; the part customers flip over to read what’s inside. It’s less flashy, but super important for compliance and trust.
Here’s what goes on your IP:
Quick reminders:
Bottom line: The IP is where you earn your customer’s trust. People want to know who made the product, what’s in it, and how to use it safely.
Once you’ve structured your label correctly, the next step is making sure it stays compliant. Even the best-designed labels can slip up on small details that cost brands big.
In 2007, the U.S. Food & Drug Administration issued a warning letter to Fusion Brands International for its “Transdermal Face-Lift” and “Eye-Lift” cosmetics. The labels featured claims such as “more effective than Botox injections” and “relaxed facial muscles.”
These statements were considered therapeutic, reclassifying the products as drugs rather than cosmetics. The labels also failed to identify the manufacturer clearly. That case isn’t just historical; for a new brand, the takeaway is this: words and placement matter.
Below is a table of frequently overlooked label errors and how to avoid them.
Once you understand the common slip-ups, the next step is building a compliance routine that scales with your brand. Start with a practical FDA labeling checklist.
Below is a focused checklist based on the U.S. Food & Drug Administration’s requirements to help ensure your label is compliant and retail-ready.
Even with the best checklist in hand, managing every compliance detail alone can be overwhelming. That’s where professional manufacturers step in, simplifying FDA requirements from the ground up.
Most beauty founders think compliance ends with a clean label, but pro manufacturers know it starts way before that. The real secret lies in the systems, documentation, and traceability they build into every batch, turning complex FDA requirements into second nature.

Here’s how experienced contract manufacturers like Respect Manufacturing make compliance a built-in part of your brand journey, not an afterthought:
When your manufacturer treats compliance as part of creation, your product isn’t just market-ready; it’s built to last.
Label compliance isn’t just about avoiding penalties; it’s about protecting brand equity as you scale. Every formula tweak, packaging update, or market expansion introduces new regulatory layers that demand precision and documentation that most startups underestimate.
The most innovative brands streamline this early by partnering with manufacturers who’ve already built compliance into their DNA. Respect Manufacturing operates at that intersection, where speed meets structure, and creativity doesn’t come at the cost of control.
When your supply chain is this disciplined, audits run more smoothly, launches move faster, and your team can focus on building what actually matters: demand.
Simplify your next launch with a manufacturer that respects every regulation as much as your ambition. Connect with Respect Manufacturing.
1. What does the FDA require on a cosmetic label?
The FDA requires every cosmetic label to include the product identity, net quantity, ingredient list, manufacturer details, and any necessary warnings.
2. What’s the difference between cosmetic and drug claims?
Cosmetic claims talk about appearance (e.g., “moisturizes skin”), while drug claims mention treatment or change in body function (e.g., “treats acne”) — which can make your product a drug under FDA law.
3. Do small or handmade beauty brands need FDA-compliant labels?
Yes. Even small or handmade cosmetic brands must follow FDA labeling rules if they’re sold commercially, online, or across state lines.
4. What happens if my cosmetic label isn’t FDA-compliant?
Noncompliant labels can lead to “misbranding,” product recalls, fines, or blocked listings on major retail and e-commerce platforms.
5. How can manufacturers help with FDA compliance?
Professional manufacturers, like Respect Manufacturing, ensure your product meets FDA and CGMP standards, from formulation to packaging and labeling.



