Ready to Upgrade Your Process Operations?
Stay compliant under MOCRA 2025. Learn FDA deadlines, facility registration rules, and how cosmetic brands can meet new safety and reporting requirements.

Since 1938, the U.S. cosmetics industry has operated under the same core law, until now. The Modernization of Cosmetics Regulation Act of 2022 (MOCRA) marks the biggest update to cosmetic safety oversight in over 80 years, giving the U.S. Food and Drug Administration (FDA) new authority over product listing, facility registration, and adverse event reporting.
According to the FDA, the average person uses 6 to 12 cosmetic or personal care products every day, from cleansers and moisturizers to sunscreens and fragrances. With such widespread daily use, consumer safety and ingredient transparency have become non-negotiable priorities for brands and manufacturers alike.
As deadlines approach for compliance, understanding MOCRA is essential. Brands, formulators, and contract manufacturers must adapt quickly to meet new record-keeping, labeling, and reporting standards without disrupting production timelines or product launches.
In this article, you’ll learn what MOCRA is, what the new FDA requirements mean for your business, key deadlines to watch, and practical steps to stay compliant while continuing to grow.
The Modernization of Cosmetics Regulation Act of 2022 (MOCRA) is a landmark U.S. law that strengthens how the Food and Drug Administration (FDA) regulates cosmetic products. It was signed into law on December 29, 2022, as part of the Consolidated Appropriations Act, marking the first major expansion of federal cosmetic regulation since 1938.
Before MOCRA, cosmetic products were largely self-regulated: the FDA could take action only after a product caused harm or was misbranded. Unlike foods or drugs, cosmetics weren’t required to be registered with the FDA or listed in a federal database. This lack of oversight often led to inconsistent safety data, limited recall authority, and patchy transparency for consumers.
MOCRA changes that by introducing mandatory facility registration, product listing, recordkeeping, and adverse event reporting, aligning the cosmetics sector more closely with the systems used for dietary supplements and pharmaceuticals.
MOCRA applies to any business that manufactures, packages, or distributes cosmetics for sale in the U.S., including both domestic and foreign entities. That means brands, private-label owners, and contract manufacturers must all ensure compliance.
Products covered include skincare, haircare, makeup, fragrance, and personal-care items, essentially, any product intended for cleansing, beautifying, or altering appearance without affecting body structure or function.
Together, these updates establish a new baseline for cosmetic safety, transparency, and accountability. Next, let's understand the detailed compliance requirements.
MOCRA introduces several new FDA oversight mechanisms that all cosmetic brands and contract manufacturers must follow. These rules are designed to improve product traceability, consumer safety, and accountability across the entire supply chain.
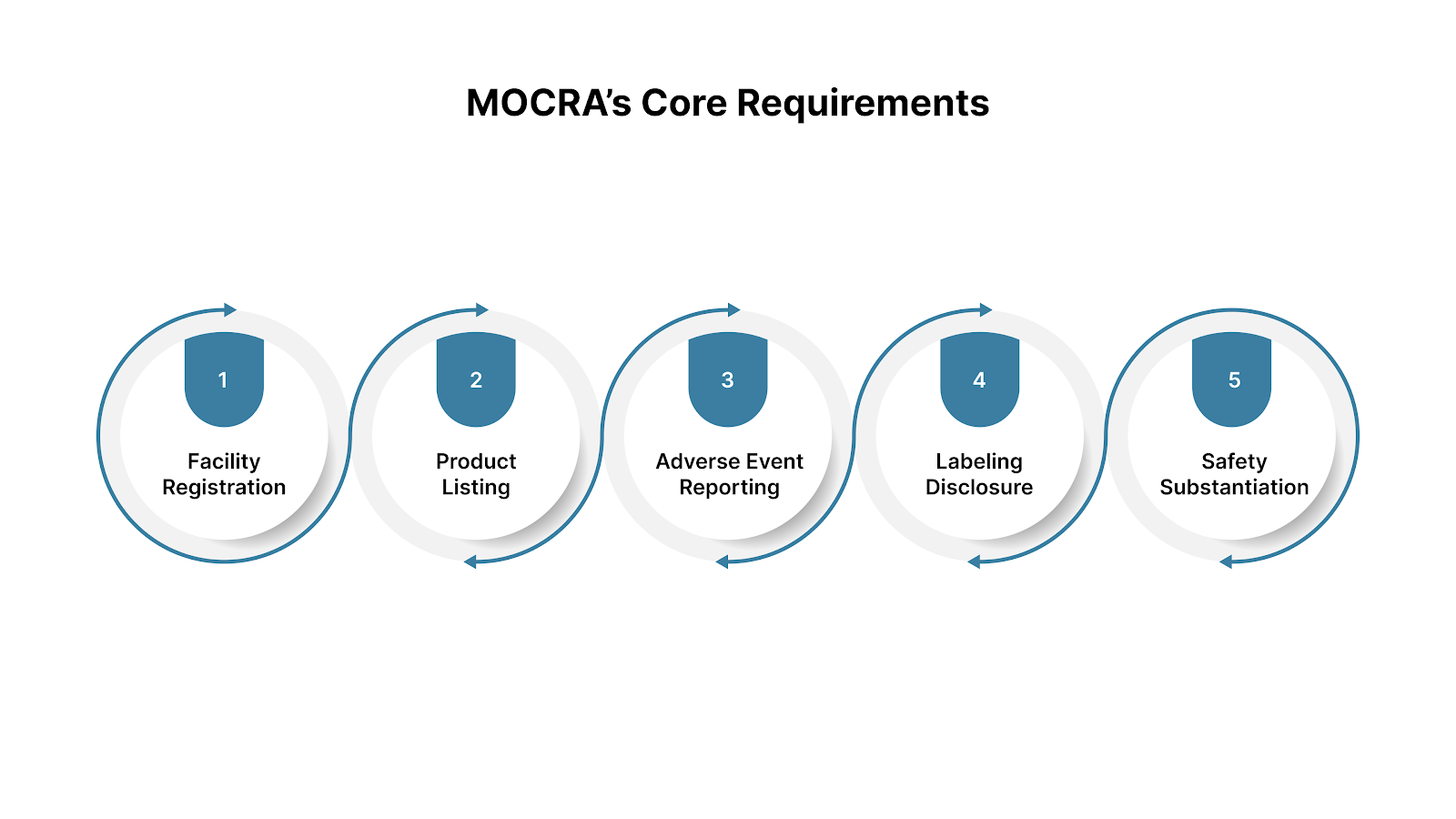
Every facility that manufactures or processes cosmetic products for distribution in the United States must register with the FDA.
Each cosmetic product marketed in the U.S. must be listed in the FDA’s Cosmetics Direct database.
If a consumer experiences a serious adverse event related to a cosmetic product (like hospitalization or a significant allergic reaction), it must be reported to the FDA.
Visibility & reporting tools: The FDA has expanded its Cosmetic Adverse Event Dashboard, allowing the public and industry to view reported serious adverse events. This added transparency means brands should monitor their own submissions and reconcile customer complaints with reported cases to identify patterns before they trigger regulatory scrutiny.
MOCRA mandates that product labels now include:
Brands must maintain documentation proving each product’s safety under labeled use. This includes ingredient data, stability studies, and any relevant toxicological reports.
Together, these requirements signal a new era of accountability, ensuring cosmetics are produced, labeled, and monitored with the same rigor consumers expect from any FDA-regulated product.
Once you know what MOCRA mandates, it’s time to understand when and how those requirements take effect. The following section outlines every key deadline, renewal cycle, and submission method you’ll need to stay compliant in 2025.
Because MOCRA represents the most substantial reform in U.S. cosmetics oversight since 1938, understanding the FDA’s phased compliance dates is critical for every brand and contract manufacturer. The FDA has issued detailed guidance outlining registration, listing, and reporting deadlines to ensure a smooth transition into the new regulatory framework .
All submissions are managed through the FDA’s Cosmetics Direct portal, the online system for:
To access Cosmetics Direct, brands and CMOs must:
With the key dates and filing process clear, let’s turn to implementation. The following checklist translates MOCRA’s legal requirements into practical, day-to-day actions that help you stay organized and audit-ready.
Meeting MOCRA requirements can feel overwhelming, especially for fast-growing beauty and skincare brands that rely on external partners. Below is a practical roadmap, built around real operational steps, that helps both brand owners and contract manufacturers prepare efficiently.
The first step is determining whether your products are regulated as cosmetics or drugs under the FDA definitions.
If your product claims to treat or alter the body’s structure or function (e.g., acne or SPF), it may fall under drug regulations instead. Brands must also designate a Responsible Person, the company listed on the product label, who ensures all submissions and reports are filed correctly.
Every domestic or foreign facility that manufactures, processes, or packs cosmetics for U.S. distribution must be registered with the FDA via the Cosmetics Direct portal.
Each cosmetic SKU marketed in the U.S. must be listed with the FDA within 120 days of product launch, and updated annually . This submission includes the product name, ingredients, the Responsible Person contact, and facility reference numbers.
Example: A skincare brand launching a new vitamin C serum must submit its listing within four months of retail launch, even if manufactured by a contract partner.
MOCRA requires Responsible Persons to report serious adverse events (SAEs) to the FDA within 15 business days of first awareness. Reports must include the product’s label, event description, and any medical details provided. Records must be kept for six years.
Train customer-care or QA teams to log complaints in a centralized system that feeds into your CMO’s documentation workflow.
MOCRA also mandates brands to maintain safety substantiation demonstrating that each cosmetic is safe under labeled use. This typically includes:
Every product label must now include:
Brands should review artwork during the next production cycle to integrate these updates.
Set up quarterly or semi-annual compliance reviews with your manufacturing partner. Audit registration statuses, labeling, record retention, and SAE log completeness. Regular reviews show FDA readiness and help prevent last-minute compliance scrambles.
Working with a turnkey partner like Respect Manufacturing ensures your facility documentation, quality testing, and regulatory records stay aligned, saving you costly re-work later.
Compliance doesn’t stop at the brand level; contract manufacturers play a critical role in meeting FDA expectations. Here’s how CMOs can align their operations, documentation, and client agreements with MOCRA’s standards.
While MOCRA primarily assigns regulatory responsibility to the “Responsible Person” (usually the brand owner), contract manufacturers (CMOs) play a pivotal role in ensuring compliance. Under the new framework, FDA scrutiny extends to both brand owners and manufacturing partners, meaning every CMO must align its processes, documentation, and contracts with MOCRA’s standards.
Each manufacturing contract should clearly outline compliance with ownership. Clauses should specify:
Example: A small skincare brand may rely on its CMO to maintain safety testing records, but must still be listed as the Responsible Person on FDA filings. Transparent agreements prevent confusion and potential enforcement issues later.
Under MOCRA, both brands and CMOs must be able to produce accurate documentation during FDA inspections. This includes:
CMOs should embed MOCRA requirements into their Quality Management System (QMS) by:
Because MOCRA now mandates label-based Responsible Person contact details, contract manufacturers must coordinate with brand owners to ensure packaging is FDA-ready. CMOs should maintain updated product lists and ensure all data aligns with Cosmetics Direct submissions.
Even with strong systems in place, many companies still stumble over MOCRA’s finer details. Let’s look at the most common compliance pitfalls and how to avoid costly enforcement risks as FDA oversight intensifies.
Even though MOCRA’s new regulations were designed to strengthen consumer safety, many brands and manufacturers are still struggling to interpret and operationalize the details.
The FDA has emphasized that, while enforcement is rolling out gradually, non-compliance will carry real consequences, especially as registration, listing, and safety data audits become more routine.
FDA recall authority (new enforcement power): Under MOCRA, the FDA now has authority to mandate recalls of adulterated or misbranded cosmetic products, a major shift from the previous voluntary recall system.
True long-term compliance comes from partnering with a manufacturer that’s already operating at FDA standards, not scrambling to catch up. Here’s how Respect Manufacturing bridges that gap.
For many growing skincare and personal care brands, MOCRA compliance can seem challenging. Registering facilities, tracking batch data, maintaining safety documentation, and updating labels all require FDA-grade systems, something most early-stage or mid-market brands don’t have in-house.
Even established companies risk non-compliance if they work with smaller, non-registered manufacturers that lack proper documentation, testing, or traceability.
Respect Manufacturing serves as a true turnkey, MOCRA-ready manufacturing partner, designed to help brands meet FDA compliance standards without operational challenges. Every product is produced in an FDA-registered facility that operates under Current Good Manufacturing Practices (CGMP), the same foundational system referenced by MOCRA’s new quality-control expectations.
This ensures each step, from formulation to packaging, meets FDA documentation and traceability requirements.
Whether you’re a startup launching your first line or an established D2C brand scaling to retail, Respect Manufacturing provides the infrastructure, documentation, and testing protocols needed to stay safe, compliant, and audit-ready under the FDA’s new cosmetics framework.
MOCRA has reshaped how cosmetics are regulated in the U.S., ushering in an era of transparency, traceability, and shared responsibility between brands and manufacturers. From facility registration and product listing to safety substantiation and adverse event reporting, compliance is now a continuous process, not a one-time task. For brands looking to build long-term trust with consumers and retailers alike, meeting these standards isn’t just about avoiding penalties; it’s about strengthening brand credibility.
As the FDA increases enforcement, the safest path forward is working with partners who are already prepared.
Respect Manufacturing has built its entire operation on the same principles that MOCRA now enforces. By aligning FDA registration, CGMP compliance, and turnkey formulation-to-packaging services under one roof, we help brands stay compliant while scaling quickly and confidently.
If your brand is preparing for MOCRA compliance, connect with Respect Manufacturing today. Our team can help you develop, test, and produce MOCRA-ready cosmetics that meet both your creative vision and the highest U.S. compliance standards.
Stay compliant. Stay confident. Build with Respect.
Yes. MOCRA applies to both U.S. and international cosmetic companies that sell products in the American market.
Foreign manufacturers must register their facilities with the FDA and list each product marketed in the U.S., just like domestic producers. The law ensures imported cosmetics meet the same safety and labeling standards as locally made products.
Any facility, domestic or foreign, that manufactures or processes cosmetics for U.S. distribution must register with the FDA. This includes contract manufacturers, private-label producers, and brand-owned facilities. Facilities must renew registration every two years and keep information up to date.
The Responsible Person is the company listed on the product label, typically the brand owner, distributor, or manufacturer, who is legally responsible for ensuring compliance with MOCRA. They handle FDA submissions, maintain product safety documentation, and report any serious adverse events.
A serious adverse event includes any health-related issue that results in hospitalization, infection, disability, or significant disfigurement (e.g., chemical burns or serious allergic reactions). Responsible Persons must report these events to the FDA within 15 business days of becoming aware and keep related records for six years.
Under MOCRA, the FDA can suspend a facility’s registration, making it illegal to sell or distribute cosmetics from that site. The agency can also issue warning letters, product seizures, or mandatory recalls if products are deemed unsafe or mislabeled. Beyond legal penalties, non-compliance can damage brand reputation and cause costly market withdrawals.



