Ready to Upgrade Your Process Operations?
Launch your successful nutrition supplement business with our 8-step guide. Learn about compliance, R&D, and choosing a CGMP-certified manufacturing partner.

The wellness and health sector offers founders a massive opportunity to build impactful brands. As consumers become more proactive about their health, the demand for targeted products like vitamins, proteins, and specialty ingredients continues to rise.
In fact, the global dietary supplements market is projected to grow from USD 93.46 billion in 2024 to USD 182.52 billion by 2032, demonstrating a CAGR of 8.73% during that period.
However, entering this highly regulated industry presents significant hurdles. The successful launch of a nutrition supplement business hinges not just on a great idea, but on flawless execution and a trustworthy production partner.
In this article, we break down the necessary steps into a practical 8-point framework designed to guide founders from concept to market-ready product, setting up your nutrition supplement business for long-term growth and compliance.
Launching a new supplement line involves more than mixing ingredients and designing a label. It needs a strategic approach for product development, legal compliance, and manufacturing. Here are the eight essential steps to guide your journey:

Before investing in formulation, you must define precisely who your customer is and what specific health problem you solve. A vague target like "health-conscious adults" makes marketing difficult and product development unfocused.
Action Steps:
Below is a nutrition supplement business niche evaluation framework:
Understanding your competitive landscape helps you position your brand effectively. Research both direct competitors (other supplement brands) and indirect competitors (whole food alternatives or lifestyle solutions).
Key Analysis Areas Include:
Tools for In-Depth Competitor Research:
To properly size up your competition, move beyond simple Google searches. Use the following:
Identify what makes successful competitors stand out and look for opportunities to differentiate through superior ingredients, more transparent sourcing, or more engaging brand storytelling.
Your product formulation transforms your business concept into a tangible offering. Key considerations include:
Consider these formulation options and requirements:
Start with a focused product range rather than numerous SKUs. This ensures quality control, simplifies inventory management, and allows for more targeted marketing as you establish your brand.
Struggling to align your formulation with FDA regulations and market demand? Contact Respect Manufacturing’s formulation consultation and R&D experts to de-risk your product development.
Selecting the right production approach significantly impacts your startup costs and operational complexity. Consider these options:
For most emerging brands, contract manufacturing provides the ideal balance of custom formulation capabilities without the massive capital investment required for in-house production.
This model offers access to expert formulators, established quality control systems, and scalable production capacity.
Regulatory compliance forms the foundation of a legitimate supplement business. Key requirements include:
Understanding that cGMP represents the quality standards enforced by the FDA is crucial for compliance.
A detailed business plan serves as your roadmap to success and is essential for securing financing. Your plan should include:
Your brand identity distills your company's values and market position into visual and verbal elements that resonate with your target audience. Key components include:
Consider these packaging options:
Modern supplement brands require a strong digital presence. Consider these sales channel options:
Create informative content that establishes your brand as a credible authority, and implement search engine optimization techniques to boost your visibility.
Moving past the basic launch steps, the difference between stability and failure in the regulated health industry lies in adopting expert-level operational rigor.
While the 8 steps cover the essential roadmap to launching your nutrition supplement business, true scalability and risk mitigation lie in adopting advanced operational and quality control protocols.
This checklist moves beyond the basics, providing a strategic framework to address the complexities of the highly regulated supplement industry:
These practices address the foundational strategy and de-risking phase.
Do not just identify competitors; analyze their customer reviews. Use marketplaces like Amazon to find recurring complaints about top-selling products in your niche (e.g., "bad taste," "stomach upset," "incomplete dosage"). These complaints are your immediate market gaps and the core of your Unique Selling Proposition (USP).
Action: Define your USP as the single reason a customer should choose you over anyone else (e.g., "The only CGMP-certified fish oil that guarantees no aftertaste").
Successful brands budget for a sufficient margin to enter retail or scale paid advertising. Calculate your Cost of Goods Sold (COGS) (raw materials, packaging, labor, freight) and ensure your final COGS is no more than 20-25% of your Suggested Retail Price (SRP).
Action: Create pricing for Direct-to-Consumer (D2C) only. If you want to expand to wholesale or retail, you must build margin for distributor and retailer markups (typically a 50% discount off SRP).
Compliance starts before printing. Do not wait for the manufacturer to flag issues. Work with a legal or regulatory expert to pre-vet your product name, all website copy, and marketing language. You must only use approved "structure/function" claims (e.g., "supports immune function"), not disease claims (e.g., "cures the cold").
These practices focus on mitigating quality and compliance risk (core concerns of the ICP).
A reliable manufacturer must provide a detailed Batch Record for every production run. This document is the legal history of your product, proving the origin of every ingredient, the exact processing steps taken, and all quality control tests performed.
Action: Insist on full traceability from raw material supplier to finished goods.
Prevent major line shutdowns and product recalls by ensuring your packaging is flawless. Acceptance Quality Limit (AQL) testing must be performed on all incoming components (bottles, caps, seals, labels) before the manufacturing run. This confirms the packaging will work correctly on the automated lines and prevent leaks or defects.
Do not launch without stability data. Stability testing ensures your product retains its potency and stated claims throughout its entire shelf life (typically 18-24 months). A compliant partner will incorporate this into the R&D phase to prevent formula degradation after launch.
These practices focus on customer retention and long-term brand building.
It costs significantly more to acquire a new customer than to retain an old one. Build subscription models and loyalty programs into your D2C platform immediately.
Action: After the first purchase, focus on creating content and email campaigns that educate the customer on product usage and highlight complementary SKUs.
Leverage your manufacturing transparency. Highlight the fact that your product is made in an FDA-registered, CGMP-certified facility. Use certifications (e.g., Organic, Non-GMO) as trust signals on your packaging and website. The modern consumer demands to know where their supplements come from.
Your product is never truly finished. Systematically collect customer feedback, especially negative reviews. Use this data to refine your formulation, improve your delivery format, or inform your next product extension, constantly maintaining an edge over competitors.
Adhering to those advanced best practices helps you preempt failure. However, every scaling brand inevitably faces three core operational hurdles that threaten quality and speed.
Even with a well-defined plan, brands scaling in the nutrition supplement business face predictable obstacles.
A common risk when switching manufacturers or working with partners who rely on outdated equipment is batch-to-batch variation in quality and potency.
Tired of juggling multiple vendors? Discover how our single-source, turnkey solution can simplify your supply chain and cut your time-to-market significantly.
Juggling separate vendors for R&D, packaging, manufacturing, and logistics inevitably leads to communication delays and scheduling conflicts.
Non-compliant labeling or poor documentation can result in product recalls, fines, and severe brand damage.
What if you could sidestep these challenges altogether? The most effective strategy is to partner with a manufacturer designed to prevent them from the start.
Launching a nutrition supplement business presents significant challenges, including regulatory complexity, quality control concerns, and the substantial capital investment required for manufacturing capabilities. These obstacles prevent many promising brands from successfully reaching the market.
Respect Manufacturing eliminates these barriers by serving as your end-to-end partner for supplement manufacturing. Our turnkey solutions transform your product concept into market-ready supplements while ensuring strict compliance, consistent quality, and faster time-to-market.
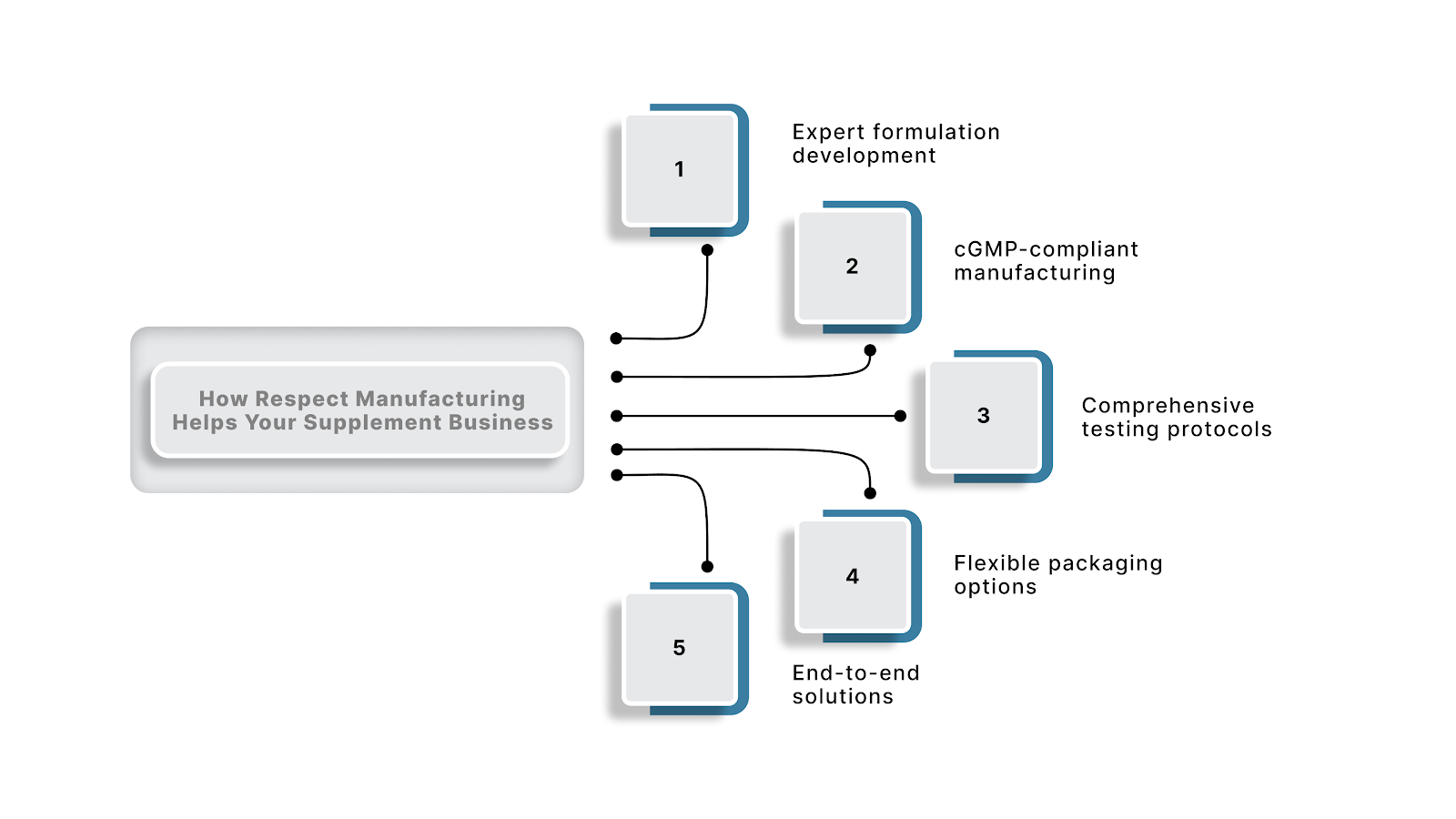
Our comprehensive services include:
By consolidating these capabilities with a single partner, we reduce your operational complexity while ensuring the quality and compliance your brand requires.
Successfully launching a nutrition supplement business requires careful planning, strategic formulation, and, most importantly, a solid commitment to quality and compliance.
By following these eight steps, from niche identification to building your sales channels, you create a defensible foundation for growth. The most crucial decision, however, remains the choice of your production partner.
To rapidly accelerate your growth and mitigate the inherent risks of scaling production, partnering with an experienced, compliant contract manufacturer is the clearest path. Respect Manufacturing’s vision is to empower wellness brands by providing guaranteed quality, transparency, and consistency at every stage of the production lifecycle.
If inconsistent quality, compliance fears, or multi-vendor delays are holding your brand back from its next stage of growth, it is time to choose a reliable, turnkey partner.
Contact Respect Manufacturing today to schedule a consultation and discover how our CGMP-certified, end-to-end solutions can turn your scaling challenges into market dominance.
Startup costs vary based on your production model and scale. While establishing your own manufacturing facility requires substantial investment, partnering with a contract manufacturer significantly reduces upfront costs.
Typical expenses include business registration, product development, initial production runs, brand development, and marketing.
The timeline from concept to market typically ranges from 3-6 months, depending on formulation complexity and regulatory requirements. The process includes product development, stability testing, packaging design, initial production runs, and quality assurance checks.
The FDA usually does not approve dietary supplements before they reach consumers, but manufacturers must ensure product safety and compliance with labeling requirements. Supplement companies must manufacture in FDA-registered facilities that follow Current Good Manufacturing Practices (cGMP).
While pre-market approval isn't required, the FDA monitors product safety and can take action against unsafe products.
Minimum order quantities (MOQs) vary by manufacturer but are a critical factor for new brands. Typical MOQs range from 5,000 to 50,000 units per product, influenced by the packaging format and ingredient complexity.
Respect Manufacturing works with emerging brands to determine cost-effective production runs that align with their business stage and market demand, offering flexibility for initial launches.
Yes, you can develop a fully custom formula. Reputable contract manufacturers like Respect Manufacturing offer both custom formulation development and private labeling of existing products.
The custom formulation process involves collaborative work between your team and the manufacturer's R&D experts to product that meets your vision, target market needs, and regulatory requirements.



